Which Best Describes a Polar Covalent Bond
If a covalent bond were to be formed between a nitrogen atom electronegativity 30 and an oxygen atom electronegativity 35 which of the following statements would best describe such a bond. Pre - Activity 1.

Which One Of The Following Best Describes A Job Cost Sheet In 2022 Cost Sheet Job Sheet
Molecular compounds are made when two or more elements share electrons in a covalent bond to connect the elements.

. The two atoms in the bond have the same electrogegativity. The bully child seems to. In a polar covalent bond the electrons are not equally shared because one atom spends more time with the.
Asked Sep 10 2016 in Chemistry by MagicCarpetRide. Two nonmetal atoms with very different electronegativities share electrons. A polar bond is a type of covalent bond in which the electrons forming the bond are unequally distributed.
A polar covalent bond is a bond formed when a shared pair of electrons are not shared equally. Answer Key on Some Items. 1 Which Best Explains Why Water Has A Much Higher Boiling Point Than Would Otherwise Be Predicted.
3 Which describes the polarity in a water molecule quizlet. Equal pull between the shared electrons D. The two atoms in the bond have an electronegativity difference of greater than 18.
Polar covalent where the oxygen atom carried the partial negative charge. Because the electronegativity values are slightly different the bonding electron pair isnt equally shared between the particles. A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed.
Equal transfer of electrons resulting in a positive and negative ion C. In short here is the summary. Polar bonds are intermediate between pure covalent bonds and ionic bonds.
The bonding type present between two metal atoms. All of the atoms electrons are shared between the atoms. Which statement below best describes a polar covalent bond.
The bonding type present in bound non-metal atoms. Electrons are shared but the shared electrons came entirely from one of the bonded atoms. Electrons are transferred completely from one atom to another.
Ionic Bonds Polar Covalent Bonds and Nonpolar Covalent Bonds. Let us consider A and B in which them is. The two atoms in the bond have an electronegativity difference of between 04 and 1 A metal bonding.
Covalent bonds exist when combining atoms give up or accept electrons. The difference between the two is that polar covalent bond is the sharing of electrons between two different elements while non-polar covalent bond is. A non-metal bonding to a non-metal.
A strong lattice of positively and negatively charged atoms held together by electrical forces. Polar covalent bonds form when two atoms share electrons equally with electrons spend the same amount of time around each atom and no partial charges are formed. Bolivianouft and 4 more users found this answer helpful.
A molecule contains two atoms. For instance polar covalent bonds typically from among hydrogen and the other non-metal. Polar covalent bonding is a type of chemicalbond where a pair of electrons is unequally shared between two atoms.
The atoms valence electrons are shared between the atoms. How molecular compounds are formed. Polar covalent bonds are usually formed between two nonmetal atoms having different electronegativities.
Have you ever seen two children play and one child acts like a bully toward the other child. 2 Why are the freezing and boiling points of water higher than would be expected for a compound of its molecular makeup. A a very electronegative atom and a weakly electronegative atom are covalently bound.
4 What best explains why water has high solubility. If the electronegativity difference between two different elements is 23 which type of bond is likely to form when atoms of those elements chemically combine to form a compound. Unequal transfer of electrons resulting in a positive and negative ion.
This is due to one of the elements having a higher electronegativity than the other. The covalent bond formed between two atoms in molecules whose electronegative difference exists is known as a polar covalent bond. Which of the following describe a polar covalent bond.
Explanation of Polar Covalent Bond. The atom which tends to attract these shared electrons or more precisely speaking the electron density of the bond towards itself is said to be electronegative. Which of these best describes a polar covalent bond.
Valence electrons are transferred from one atom to the other. Polar covalent bonds form among two non-metal atoms that have sufficiently different electronegativities from one another. Which best describes a polar bond.
The atoms valence electrons combine to form a network of bonds. Lets look at water H 2 0. The bonds in bao are best described as an ionic compound is formed when there is a reaction between the elements which of the following best describes a metallic bond.
Typically non-metals tend to share electrons make covalent bonds and thus form molecular. For example the bond between H and F in an HF molecule is a. Polar Covalent Bonds.
The equal sharing of two electrons between adjacent atoms. This causes the molecule to have a slight electrical dipole moment where one end is slightly positive and the other is slightly negative. C two very electronegative atoms undergo ionic bonding.
Quantum Numbers Atomic Orbitals and Electron Configurations. A covalent bond whose shared pair of electrons tend to lie closer to one of the two atoms forming the bond is called a polar covalent bond. It has no units simple it is a tendency.
They form when the electronegativity difference between the anion and cation is between 04 and 17. Which of the following best describes a polar covalent bond. In other words the electrons spend more time on one side of the bond than the other.
Which of the following statements best describes a relatively polar covalent bond. Unequal pull between the shared electrons B. List all that apply.
Which statement correctly describes a covalent bond. B two very electronegative atoms are covalently bound. A covalent bond that has an unequal sharing of electrons and the electronegativity difference is within the range 01-2 is called a polar covalent bond.
- the ability of an atom to attract electron in a covalent bond. B Describe a nonpolar covalent bond and give an example.

Help Students Understand How To Draw Lewis Structures Understand Molecular Geometry And The Polar Nature Of Ion Molecular Shapes Molecular Geometry Molecular
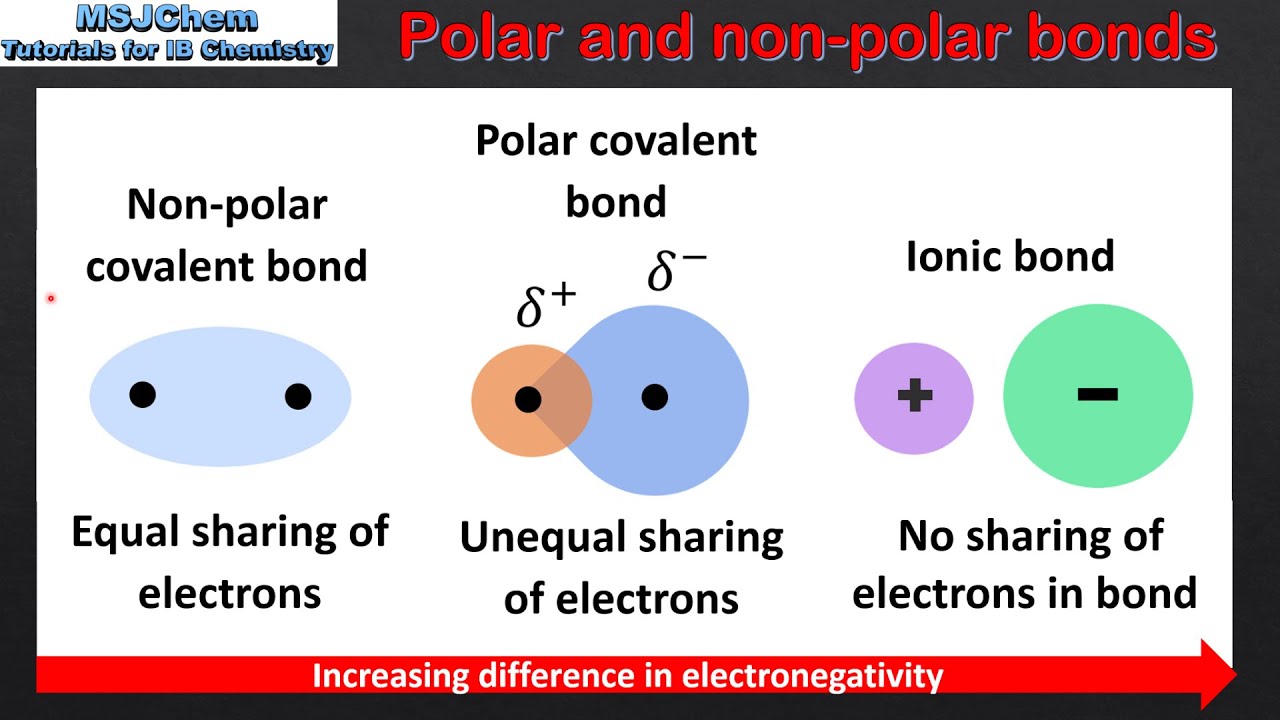
8 4 Polar Bonds And Molecules Chemistry Quizizz

Bonding Covalent And Ionic Bonds Shmoop Chemistry Covalent Bonding Teaching Chemistry Ionic Bonding
No comments for "Which Best Describes a Polar Covalent Bond"
Post a Comment